Cotinine Testing
Workers who smoke cigarettes are more expensive to employ. Why? Because smoking negatively affects the health of smokers which increases the cost of health care insurance—to the tune of $193 billion between 2000 and 2014. Consider that lost productivity accounted for $97 billion and health care expenditures accounted for $96 billion. Wow! No wonder employers are interested in hiring workers who do not smoke and the Affordable Care Act, commonly known as Obamacare, actually paves the way for employers to screen applicants and employees for Cotinine, a metabolite of Nicotine that proves that a person has been smoking.
Whether you are considering cotinine testing for employment or as part of a corporate wellness program, to screen job applicants or deter smoking as part of a cessation program you need to consider testing for cotinine.
Lab-Based Saliva Cotinine Test
Tobacco use is the single most preventable cause of disease, disability, and death in the United States with an estimated 443,000 premature deaths each year – another 8.6 million people live with serious illness caused by smoking.
In 1984, Surgeon General C. Everett Koop presented the first of a series of reports revealing the health consequences of tobacco use including involuntary exposure. While the United States has not overcome the challenges of the the “smoke-free” society that Koop hoped for by the year 2000, industries across the country now recognize the importance of testing.
Lab-based Saliva Cotinine Test will give you an exact measurement of cotinine which you can access on an Internet system 24/7.
Shelf life of 15-18 months
1. Why test for cotinine and not nicotine?
Cotinine is the first-stage metabolite of nicotine. Because the window of detection for nicotine is relatively short (approximately 2 hours), cotinine extends the window of detection for several days and is the preferred method of screening for tobacco use.
2. Which sample types can be tested for cotinine?
Urine, serum and oral fluid samples. Concentrations will vary depending on sample type but each provide comparable positive rates.
3. Will a second-hand smoke exposure produce a positive result?
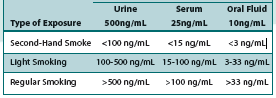
No. In numerous studies, cotinine detection levels have been examined across specimen types and exposure rates for passive exposure. Cotinine tests have been clinically designed to accomodate for the minute traces commonly found from second-hand exposure when compared to levels found in smokers. The cutoff value of these tests are set to properly distinguish passive exposure from true smokers. Below are some common examples of testing outcomes:
Test Cutoffs:
As you’ll note, the lower concentration of second-hand smoke is well below the test cutoff, and therefore would not be reported as a positive. In addition, urine tests are typically much higher than serum or oral fluid as a result of the higher concentrations of cotinine found in urine. However, the positive rates across sample types remains relatively similar.
4. Can the cotinine level be used to determine the type and volume of tobacco used?
No. While it’s true that the more a person smokes, the higher the cotinine level is likely to be, a number of contributing factors influence testing outcomes such as:
•
Size of the individual
• Percentage of body fat present
• Rate of metabolism
• Hydration state of the individual
• Type of cigarette/cigar/pipe smoked or tobacco chewed
• Smoking style
• Elapsed time from smoking to testing
• The pH of the urine (if urine testing is conducted)
5. What practices or other substances may be used that could generate a positive cotinine test result?
Certain cultures found in India or Asia are known to chew and ingest betelnuts. The preparation of this product is commonly mixed with tobacco producing a positive cotinine test result.8 Additionally, certain vegetables contain small concentrations of nicotine like eggplant or tomatoes. Only copious amounts consumed could trigger a cotinine positive test result.3 Finally, cotinine may also be found amongst tobacco workers who strip the tobacco leaves from their stalks. Absorption through the skin can produce a positive result even if the worker does not in fact smoke. There are a few other examples where a positive result may occur from a non-smoker but theses circumstances are very rare. Investigation of the individual’s lifestyle or occupation typically uncovers these anomalies.
6. Which testing method is the most accurate?
The different cutoffs in the three test types generate similar sensitivity and specificity for the cotinine tests with similar windows of detection. The better question to ask is which method best suits my testing purposes. If a flexible, non-invasive format is what you need for your testing program, then the lab-based saliva collection device offers the same performance as our urine and serum tests while providing optimal versatility in the cotinine testing industries.
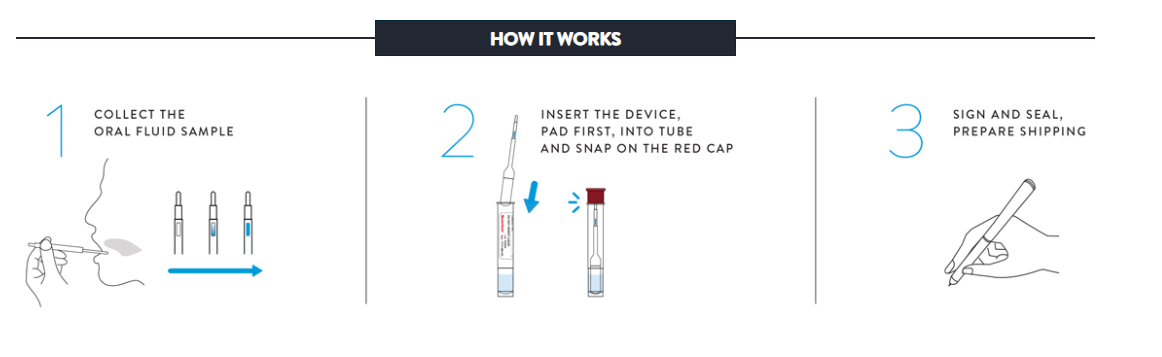
7. How is the sample collected?
The donor controls his or her own sample under direct visual supervision. The “collector” really is an observer and not in the “chain-of- custody” – the process most often legally challenged. If the donor wants to challenge the collection, the only person to challenge is the donor. The entire process can be completed anytime – anywhere and takes just minutes to administer.
8. What is the stability of the sample and how is it shipped?
Specimens stored in the Oral Fluid Specimen Vial may be kept at 39ºF to 98ºF for up to 21 days (including the time for shipping and testing). Shipping of the specimen is generally done via overnight courier as part of your arrangement with the laboratory.
9. How is the sample analyzed?
Oral fluid samples are screened in a certified laboratory using tests which use enzyme immunoassay technology (EIA), proven reliable for routine cotinine testing. Studies have shown that there is good agreement between test results and confirmation testing by gas chromatography/mass spectrometry (GC/MS or GC/MS/MS). Your laboratory can help you set up confirmation strategies in accordance with your testing policy.
10. What is the detection window for oral fluid?
Just like traditional urine drug testing, the window in oral fluid testing is similar for cotinine. What we have found is that oral fluid screening identifies recent usage that can be missed by urine testing. Oral fluid samples provide a close match to drug levels in blood, identifying donors that are under the influence at the time the sample is collected. Since the testing system detects the nicotine metabolite, the window of detection in oral fluid starts almost immediately after tobacco use and can last up to about three days.
11. Can oral fluid be adulterated?
A wide range of compounds and techniques available today have been studied, and has not identified any that can alter the results of the test. Furthermore, the risk of the donor introducing any compound into the vial is highly unlikely since every collection is directly and easily observed. These facts will cut all costs associated with adulterant and dilution testing common with urine testing.
12. What are the economic advantages of using oral fluid?
Oral fluid drug testing, with its “anywhere / anytime” collection process, results in significantly lower costs associated with collections, scheduling fees, and lost time on the job. Oral fluid collections reduce the labor costs for gender-specific collectors and shy-bladder syndrome (can’t go), and eliminate dilution testing – all of which contribute to the higher costs of urine testing.
13. How soon can you expect your results?
The lab receives samples via overnight courier. Testing is performed the day samples arrive and positive or negative results are reported within 24 hours. Should confirmation testing be required, an additional 24 to 48 hours should be expected.
14. Is oral fluid a biohazard?
No. OSHA does not consider oral fluid collection hazardous. In addition, oral fluid specimens are not subject to the same handling and disposal procedures that are issues with other bodily fluids.
15. Who uses OraSure?
Millions of Collection Devices have been utilized nationally and internationally by large and small organizations over the past 20 years. These include insurance companies for insurance risk assessment, hospitals and businesses for health and wellness programs, as well as companies conducting research or clinical trial applicant evaluations.
16. How is training done?
Test administrators can be trained and certified in about an hour. This can be done online.
References: 1) CDC. Tobacco Use–Targeting the Nation’s Leading Killer, 2011. 2) National Library of Medicine. The C. Everett Koop Papers. Tobacco, Second-Hand Smoke, and the Campaign for a Smoke-Free America, http://profiles.nlm.gov/ps/retrieve/narrative/QQ/p-nid/85, accessed 9/21/11. 3) Benowitz, N. Cotinine as a Biomarker of Environmental Tobacco Smoke Exposure. Epidemilogic Reviews, 1996; 18(2):188-204. 4) Zuccaro, P. et al. Serum Cotinine as a Marker of Environmental Tobacco Smoke Exposure in Epidemiological Studies: The Experience of the MATISS Project. European Journal of Epidemiology, 2003; 18(6):487-92. 5) Jenkins, R. et al. Personal Exposure to Environmental Tobacco Smoke: Salivary Cotinine, Airborne Nicotine, and Nonsmoker Misclassification. Journal of Exposure Analysis and Environmental Epidemiology, 1999; 9(4):352-63. 6) Haufroid, V. et al. Urinary Cotinine as a Tobacco-Smoke Exposure Index: A Minireview. International Archives of Occupational Environmental Health, 1998; 71:162-68. 7) OraSure Technologies, Inc., Auto-Lyte® Cotinine EIA Serum, Auto-Lyte® Cotinine EIA Urine, Cotinine Urine Micro-Plate EIA, Cotinine Serum Micro-Plate EIA, OraSure® Oral Fluid Cotinine Micro-Plate EIA Package Inserts. On file at OraSure. 8) Hu, Chiung-Wen, et al. High-Throughput Siultaneous Analysis of Five Urinary Metabolites of Areca Nut and Tobacco Alkaloids by Isotope-Dilution Liquid Chromatography-Tandem Mass Spectrometry with On-Line Solid-Phase Extraction. Cancer Epidemiology, Biomarkers & Prevention, 2010; 19(10):2570-2581.
Instant Saliva Cotinine Test
Features and Benefits:
- Accurately screen for cotinine--the primary metabolite of nicotine
- Detects up to 1-2 days after nicotine use
- Qualitative detection of cotinine in oral fluids at 30 ng/mL cutoff level
- Simple collection and testing procedure
- Results in as little as 10 minutes
- Long shelf life at room temperature storage
- Integrated internal procedural control
- Gives pass/fail results
- Shelf life of 15-18 months
Encouraging Tobacco-cessation using outcome-based cotinine testing -- Quest Diagnostics
As company-sponsored wellness programs strive to encourage improving health outcomes among employees, many employers provide financial benefits for employees who pass certain screening measures. It is especially common for employers to place Outcome-Based Rewards™ around financial incentives for employees who do not use tobacco products or who are trying to quit using tobacco products. Some employers rely on their employees to accurately self-report their smoking status, but studies have shown that approximately 20% of employees that self-report as “non-tobacco users” actually test positive for the presence of nicotine. Many employers are now testing cotinine to more accurately assess employee tobacco use.
What is cotinine?
Cotinine is the major metabolite of nicotine, formed shortly after nicotine enters the body. A person can be exposed to nicotine through use of or exposure to tobacco products (cigarettes, chewing tobacco, e-cigarettes, nicotine patches, etc.). Cotinine can be measured in the laboratory from salivary, blood or urine samples. Cotinine is the preferred method of testing for nicotine exposure, as cotinine has a longer half-life than nicotine. For example, the half-life of nicotine in a person’s blood is 30 minutes to three hours, while the half-life of cotinine is 15-20 hours.
What yields a positive cotinine result?
Quest Diagnostics Health & Wellness utilizes a specific immunoassay to test for the presence of cotinine in a blood specimen. In order to test positive for cotinine, a level of 10ng/mL or greater must be present in the specimen. This level is set 20 to 30 times higher than what is expected for non-users exposed to second-hand smoke, ensuring an accurate depiction of tobacco use.
Can someone who does not use tobacco test positive for cotinine?
Although our studies have not found any substances capable of causing false-positives, in rare circumstances, some employees with a positive result claim they are not using tobacco products. Quest Diagnostics Health & Wellness believes it is appropriate for employers to have an appeals process and plan in place for employees wishing to challenge the result of their cotinine result, or for employees who are in the process of quitting tobacco with the use of nicotine patches or gum. The suggested appeals processes can include cotinine retesting or a signed physician affidavit stating the employee does not use tobacco or is in the process of quitting. Persistent concerns about a repeated false-positive immunoassay result can be excluded, if warranted, by confirmatory testing using liquid chromatography/tandem mass-spectroscopy (LC/MS/MS).
Resources: 1, 2. “Factsheet – Cotinine.” Centers for Disease Control and Prevention. 2015.